
SCCS preliminary assessment on Thiomersal and Phenylmercuric salts as preservatives in cosmetic products.
The SCCS has issued a preliminary safety assessment on thiomersal and phenylmercuric salts – understand the scientific concerns and potential regulatory consequences... Read more.
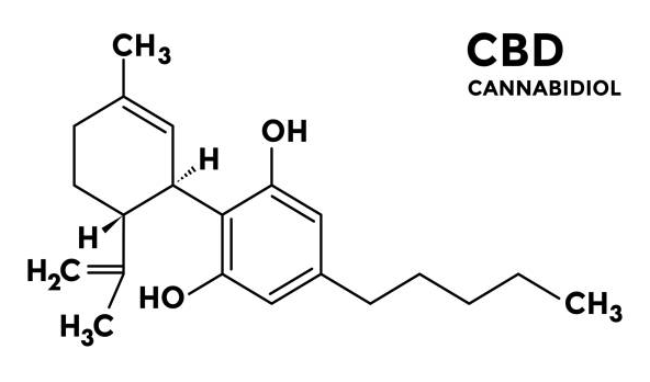
SCCS preliminary opinion on Cannabidiol (CBD) used in cosmetic products.
Is CBD allowed in cosmetics? Get clarity on the regulatory status of CBD worldwide, key risks, and what brands must know before placing products on the market.... Read more.

Canada implementation of new labeling requirements for allergens
Canada is strengthening allergen labeling rules for cosmetics – understand what’s changing, who is impacted, and how to ensure compliant labels before enforcement... Read more.
ECHA proposal to ban octocrylene in cosmetic products
ECHA Proposal to Ban Octocrylene: Public Consultation Now Open Home Information Letter 144 ECHA proposal to ban octocrylene in cosmetic products Octocrylene, a UV... Read more.

Publication of the 22nd Adaptation to Technical Progress: Updates to the CLP Regulation (EU 1272/2008)
Publication of the 22nd Adaptation to Technical Progress amending the annexes of the CLP regulation EU 1272/2008 Home Information Letter 135 October 2024 –... Read more.

Publication of the 22nd Adaptation to Technical Progress: Updates to the CLP Regulation (EU 1272/2008)
The updated CLP Regulation (EU) 2024/2865 introduces new hazard classifications, stricter labelling rules, and digital innovations, impacting suppliers and manufacturers... Read more.

UK Cosmetic Regulation Update: Alignment with EU Standards
On October 3rd, the UK notified the WTO of a draft regulation aligning its cosmetics rules with EU standards. The proposed “Cosmetic Products (Restriction of Chemical... Read more.

Publication of the 22nd Adaptation to Technical Progress: Updates to the CLP Regulation (EU 1272/2008)
The European Commission has notified the WTO of the upcoming Omnibus VIII Regulation, expected for adoption in Q1 2026 and enforceable from May 1, 2026. This regulation... Read more.

Cosmetic Labels: Navigating Key Differences
Cosmetic labels are more than design—they ensure safety, transparency, and compliance. But labeling rules differ worldwide. From the USA’s FDA to the EU and... Read more.

Canada data collection notice concerning PFAS
In August 2024, Canada launched a PFAS data collection initiative under the Chemicals Management Plan. Companies handling listed PFAS in 2023 must report details—including... Read more.

