Omnibus Regulation VII
The European Commission notified to the World Trade Organization (WTO) the “Omnibus VII” regulation on the 24th of June. The adoption is expected to take…

The European Commission notified to the World Trade Organization (WTO) the “Omnibus VII” regulation on the 24th of June. The adoption is expected to take…

Understanding MoCRA labeling requirements MoCRA Label Requirements: The Modernization of Cosmetics Regulation Act (MoCRA) 2022 introduces significant changes to cosmetic labeling in the USA, aiming…

Cosmetics expiration date: In the era of “Conscious beauty”, the shelf life and expiration date of cosmetics is an important issue for many brands. Here is a comprehensive compliance guide on…

Firstly, it is important to note that the US and EU cosmetics regulations are completely different! The major difference is in the spirit of the…
Regulatory Context Drug Facts serves as the labeling format and source of information for Over The Counter (OTC) products as set forth by the Food…

Introduction to Cosmetic Product Safety Report (CPSR) Understanding the requirements for selling cosmetic products in Europe and the UK is crucial for compliance. Our comprehensive…

A “cosmetics check” is essential for ensuring your cosmetic products are safe, compliant, and market-ready. At Biorius, we guide you through this process meticulously, covering…

EU cosmetic compliance Embarking on the journey of achieving EU cosmetic compliance can seem like navigating through uncharted waters. However, with the right guidance and…
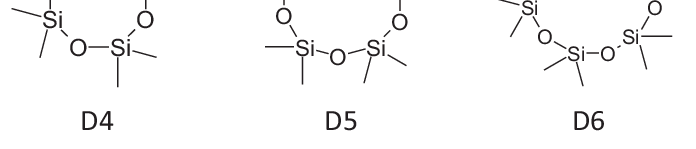
BIORIUS Information Letter 129: D4, D5, D6 REACh restrictions published The Commission Regulation (EU) 2024/1328 of 16 May 2024 on Cyclotetrasiloxane (D4), Cyclopentasiloxane (D5) and Cyclohexasiloxane…