US Beauty Trends & Regulatory Essentials
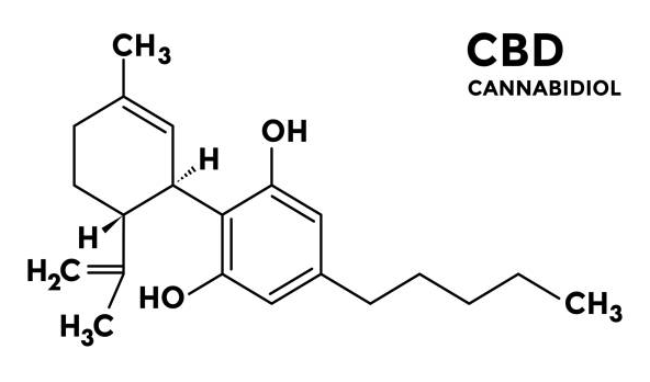
The US beauty market offers significant growth opportunities, but success requires more than strong branding. In this article, we share key insights from our presentation at Cosme Week Tokyo 2026, covering US consumer trends, the rise of clean and ingredient focused beauty, the growing influence of Amazon, and the essential regulatory requirements under MoCRA and state level laws. Discover what brands need to know before entering the US market.