Cosmetic Labels: Navigating Key Differences
September 2024: Introduction to Cosmetic Labels Cosmetic labels are more than just decorative features; they provide critical information about product safety, ingredients, and usage instructions….

September 2024: Introduction to Cosmetic Labels Cosmetic labels are more than just decorative features; they provide critical information about product safety, ingredients, and usage instructions….

August 2024: As part of the Chemicals Management Plan (CMP), Canada is collecting crucial information on chemical for guiding the prioritization, risk assessment, and risk…
July 2024: Regulatory Context Drug Facts serves as the labeling format and source of information for Over The Counter (OTC) products as set forth by…

Omnibus Regulation VII Home Information Letter 132 July 2024 – Notification of the “Omnibus VII” regulation by the European Commission The European Commission notified to…
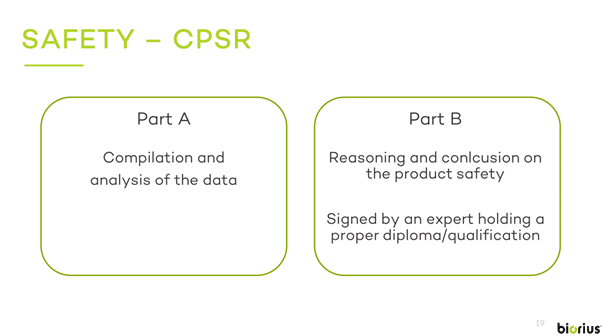
June 2024: When you apply your favorite skincare product or cosmetic, have you ever thought about the rigorous safety checks it went through before reaching…
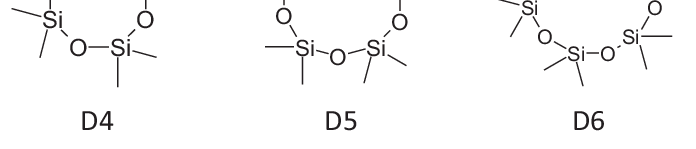
June 2024: BIORIUS Information Letter 129: D4, D5, D6 REACh restrictions published The Commission Regulation (EU) 2024/1328 of 16 May 2024 on Cyclotetrasiloxane (D4), Cyclopentasiloxane (D5) and…