Firstly, it is important to note that the US and EU cosmetics regulations are completely different!
The major difference is in the spirit of the law:
- The EU is a protective society: the key words are safety and certainty. The EU is very directive (or managerial) and leaves very little room to manufacturers by establishing a lot of restrictions/prohibitions.
- The US is a litigious society: the key words are freedom and responsibility. It is of course also forbidden to put a dangerous product in the US market and it is very important to make sure that everything is OK before selling them on the US market. But there is indeed a lot more freedom to demonstrate the safety of an ingredient with an important Toxicological work beforehand.
This would allow to put products in the US market that could not enter the EU market. However, dyes are much more limited and regulated in the US than in the EU.
Before going any further, let’s compare the geographical scope of Europe and the USA.
1. Let’s start with the old continent (Europe):
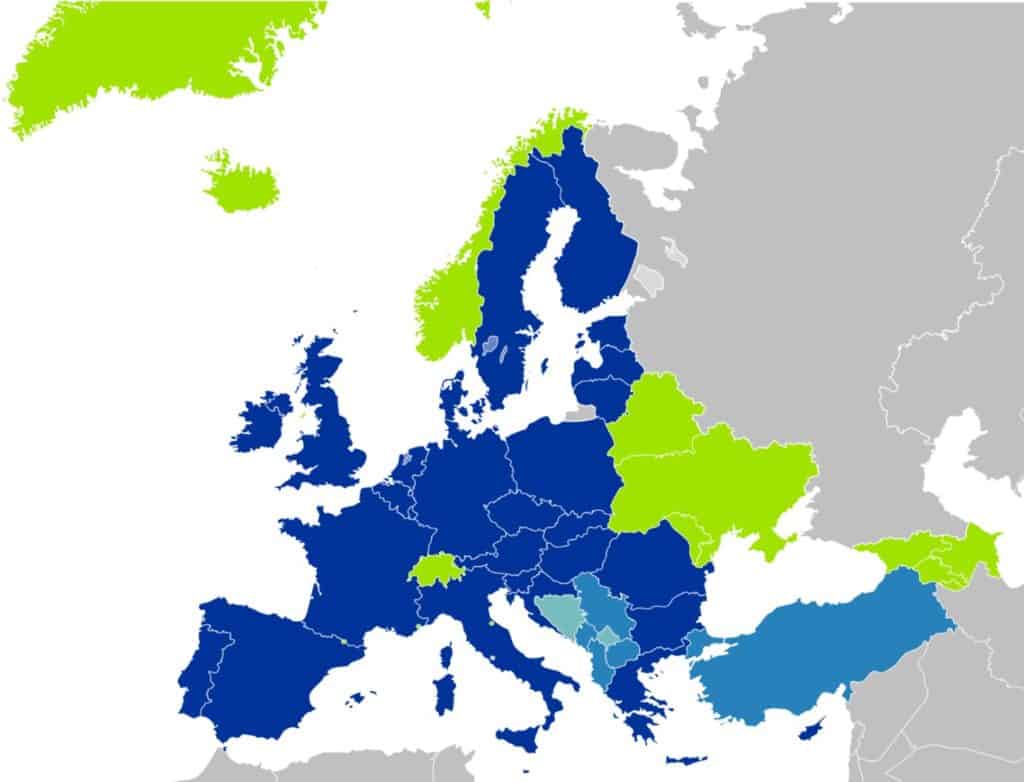
- Dark blue countries: in all these countries, the European Cosmetics Regulation applies at 100%
- Light blue + Green countries: the European Cosmetics Regulation applies with some specificities
- With Brexit, the UK has become a light blue or green country. You will find more information on this matter HERE, HERE and HERE.
From an American point of view, the EU Cosmetic Regulation may seem ridiculously long and complicated.
The EU Cosmetic Regulation is indeed and without doubt the most complicated (and longest) in the world!
If an American wants to sell his cosmetic products in Europe:
In Europe before selling anything, you must indeed make sure and confirm it will not harm anyone!
Before selling anything, you indeed have to prove and confirm to your country (and to Europe) that your products will not harm anyone!
Therefore, before being placed on the EU market, each cosmetic product indeed has to comply with the EU Cosmetic Regulation and has to have an EU Responsible Person (with offices in Europe):
- Formula Review: to check if everything is compliant (because some ingredients are prohibited) and to check which lab tests are mandatory
Some cosmetic products indeed also require additional laboratory tests (it mainly depends of the cosmetic product and the cosmetic formula):
Stability and compatibility – 3 months at 40°C
Challenge test (PET) – USP 51 (no Challenge test if no water)
Microbiological Test – USP 61 + 62
- CPSR A&B: (Cosmetic Product Safety Report) signed by a Toxicologist: a rather long and complex file of the cosmetic regulation.
- Label and claims review: clear and precise labels with certain mandatory information are of course also strictly necessary.
- PIF (Product Information File): a complete cosmetic regulatory file that contains the Formula, the CPSR A&B and the labels.
- EU CPNP (Cosmetic Products Notification Portal) notification: in other words, a notification to announce to Europe that you will sell these products in Europe.
- EU Responsible Person:
A simple and easy way to explain an EU Responsible Person is:
- Who do European countries have to call if they want to control your cosmetic product?
- Who should a consumer call if he has an unusual reaction to your cosmetic product?
Being Responsible Person comes with many responsibilities and the Responsible Person will be held responsible in the event of a non-compliance problem (it is therefore also the Responsible Person who will have to pay the fines for non-compliance with the regulations).
An EU summary: In Europe, a regulation is immediately applicable by all EU Member States without any transposition. National provisions are possible for aspects not covered by the Cosmetics Regulation (e.g. environmental issues, French legislations, German legislations, etc)
You will also find more detailed information on the EU cosmetics regulation HERE.
2. And continue with the USA:
- The FDA Federal Food, Drug, and Cosmetic Act is a very short law (1 page).
- The related Regulation (CFR, Title 21, Chapter 1, Subchapter G, Sections 700-740) is short too.
- Most of the States only apply the Federal legislation but it exists national provisions (example, California legislation)
Important udpate: On December 29th 2022, the most significant amendment to cosmetic requirements (21 U.S.C., Sec. 321-392) since 1938 was enacted: the already infamous MoCRA (Modernization of Cosmetic Regulation Act). This new piece of legislation introduces new obligations for the cosmetics industry, and we anticipate a substantial impact for cosmetic brands distributing in the USA. Beyond new regulatory provisions, MoCRA is considered as a paradigm shift in the way cosmetic products are regulated in this region. More surveillance, inspection and injunction powers are allocated to the FDA to protect public health. Among other mandates, the FDA is allowed under MoCRA to suspend the registration of a facility and order product recalls. In that sense, the way to regulate cosmetic products in the USA tends to align with the European law philosophy. Therefore, the information hereunder may not be completely up to date anymore.
If an European wants to sell his cosmetic products in America:
From a European point of view, the US cosmetics regulation is quite easy and fast. You indeed only need the following: Formula Review, TRA (Toxicological Risk Assessment) and Label and claims review.
You just have to make sure you made all the necessary to avoid a very long, painful and expensive lawsuit (which everybody would prefer to avoid at any cost of course).
Another way to say it is: you have to make sure your products are compliant in the event of an inspection of the authorities. They may indeed ask you to present all the necessary documents (Formula Review, Label and claims review, Toxicological Risk Assessment, …).
Even if your products have been approved for import into the USA, it does indeed not mean that they were approved by the US Cosmetic Regulation!
The VCRP (Voluntary Cosmetic Registration Program) registration is only an additional protection in case of problems with your cosmetic products… and it is not even mandatory in the US ! It only provides the information available on your cosmetic products and ingredients directly to the FDA (Food and Drug Administration) to allow a smoother, easier and faster control.
An US summary: The only cosmetics “complication” in the US could perhaps be related to some states (California being the most complicated state with the “California Air Resources Board”, the California Safe Cosmetic Program and the Proposition 65).
But no worries, Biorius offers a US Federal Regulatory Package and a US Federal and States Regulatory Package (to check the Cosmetic Regulation in all the US states).
You will also find more detailed information on the US cosmetics regulation HERE.
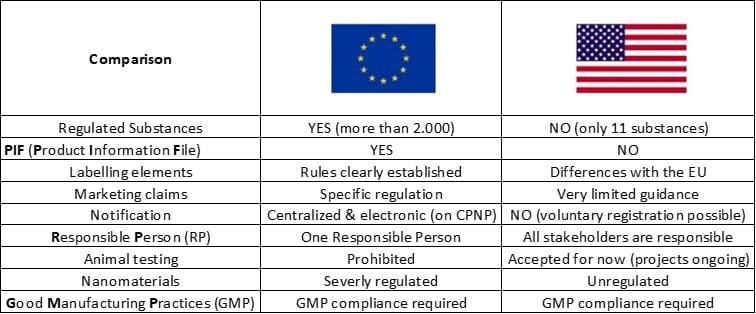
A short comparison in 1 small table:
And what about labeling rules?
A few differences between US and EU:
- Name and place of business: it can be the manufacturer, the packer or distributor
- Specific warnings to display for Aerosols, Foaming Detergent Bath Products, Feminine Deodorant Sprays, tanning products
- Net content: in the US units (ex. fluid ounces) but metric system in addition is allowed.
What about the INCI list?
- USA uses American « INCI » names
- Descending order of predominance but:
- Exception for OTC drugs: Active drug ingredients come first
- Exception for colorants: « May contain » OK, +/- not OK
- Exception for trade secrets: « and other ingredients » if the dossier is accepted by the FDA
- No obligation to list the fragrance allergens
- ‘Parfum’ and ‘Aroma’ à to be listed as « Fragrance », « Flavor » or « Flavor and fragrance »
- No specific labeling requirements for nanomaterials
- No obligation to list incidental ingredients
What about claims?
- Like in the EU, the US legislation prohibits to make claims going beyond the scope of ‘cosmetic’ definition but…
- … the US did not enforce specific legal instruments such as EU No 655/2013 and their legislation can be regarded as less stringent although requiring equivalent substantiation.
- « Claims must be truthful and not misleading ».
Conclusion of labeling rules:
The US is less prescriptive than the EU when it comes to safety assessment, but brands should be careful as a wrong action in the US leads to a class action (or lawsuit):
- Having an EU Safety assessment is a good basis to go to the US.
- It is possible to make single product label if you have a good strategy (unless the product is an OTC).
Making compliant claims both in the US and the EU is possible…if you follow the EU guidelines, you will be covered.
Recommendation for labeling rules:
Formulate a product for both regions and work with regulatory professionals to craft a single product label.
I will end this comparison by saying that Biorius can of course completely help you with the EU and US cosmetics regulations.
Get in Touch
Need a piece of advice, a quotation or answers to your questions? Contact us…
Fill in this form or contact us directly: info@biorius.com. We will answer as soon as possible!
As specialists in cosmetic Regulations for more than 15 years, Biorius offers a reliable turnkey solution for placing cosmetic products in various markets:
- First Class specialists in cosmetic regulations in Europe, the United Kingdom, the United States, and in more than 60 countries
- 50 regulatory experts, toxicologists, pharmacists, and chemists to serve you.
- A unique model that guarantees you both the fastest turnaround possible and high-quality services. Curious? Ask us to know more!
- No hidden costs: Biorius does not charge you for any question, call, or meeting.
- A best-in-class IT tool, free of charge, and saving a lot of your time.
- More than 1,500 international clients have already chosen Biorius!
- We evaluated more than 100,000 products and never had any compliance issues (fines, withdrawals from the market, etc.) in 15 years of existence.
Get in Touch
"*" indicates required fields


