May 2024: BIORIUS Information Letter 128: Update of the Canada Cosmetic Ingredient Hotlist
Health Canada has updated the Canada Cosmetic Ingredient Hotlist. It includes a part of the modifications proposed in January 23, 2023. However, some proposed updates are still subject to further consultation, as indicated in the March 14, 2024 alert.
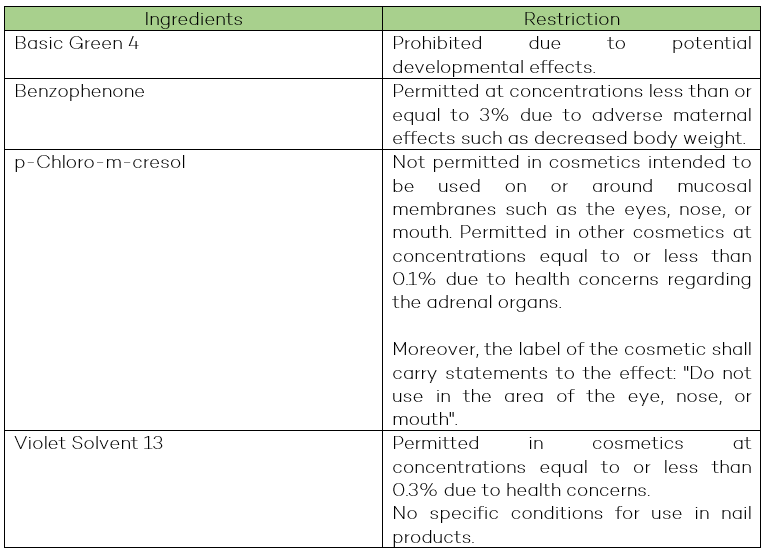
Items added to the Cosmetic Ingredient Hotlist
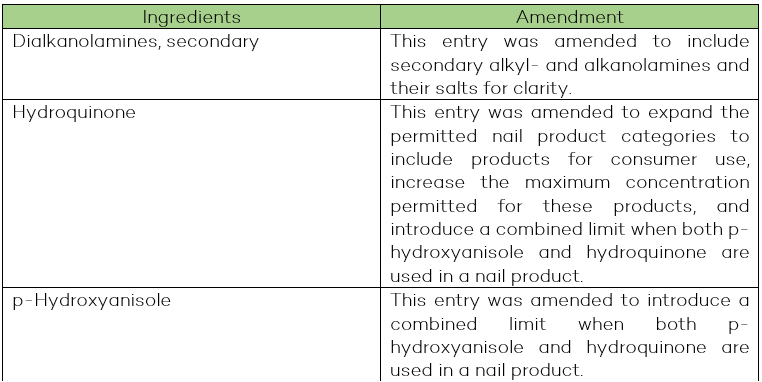
Items Amended in the Cosmetic Ingredient Hotlist
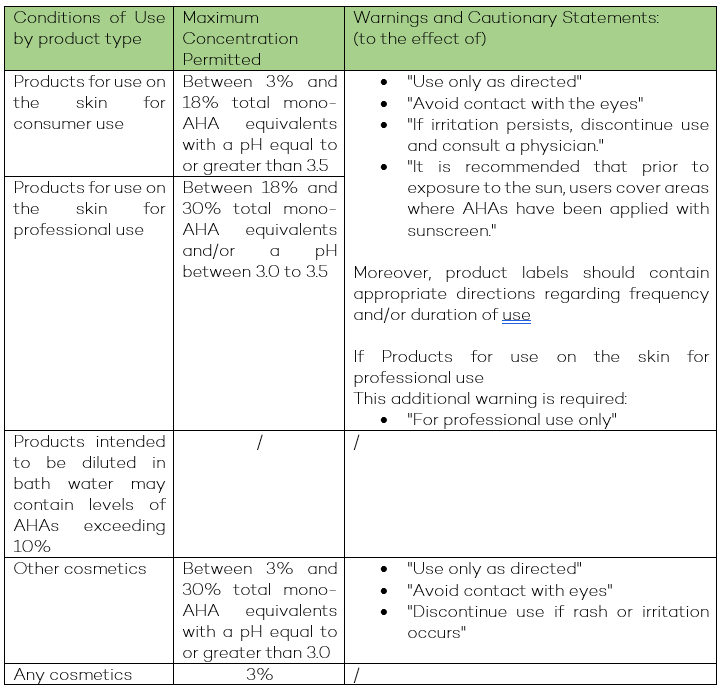
Moreover, the entries of AHAs and Talc have been revised as follows:
Alpha-hydroxy acids: This entry was amended to clarify that Polyhydroxy acids (PHAs) and bionic acids with alpha-hydroxyl groups, as well as their salts, are included. Additionally, the maximum permitted concentration for the consumer use category was increased from 10% to 18%.
Other amendments include updated warnings and cautionary statements and additional product-specific directions for safe use.
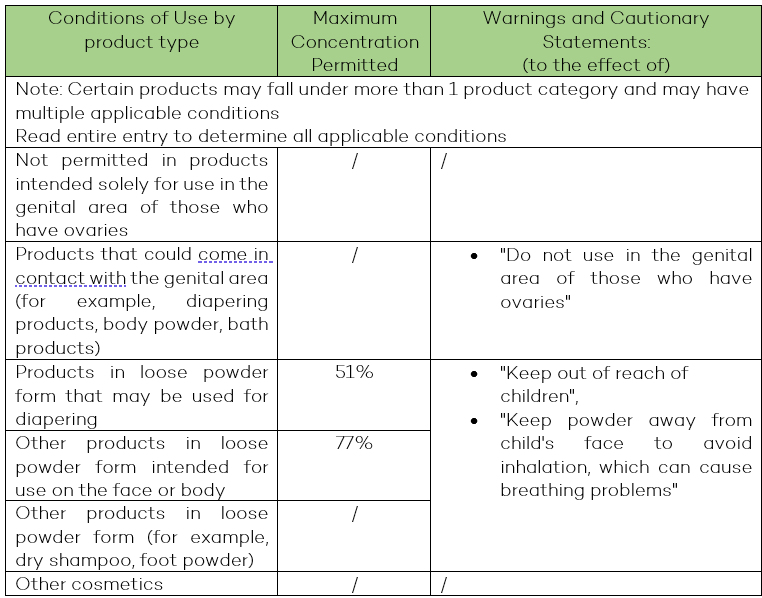
Talc: This entry was amended to help reduce chronic inhalation exposure to talc in cosmetics that may result in non-cancer lung effects, as well as genital exposure to the population with intact ovary/ovaries which may result in ovarian cancer. Cautionary statements related to acute inhalation risks were also adjusted to include all loose powder products.
More information available HERE.


