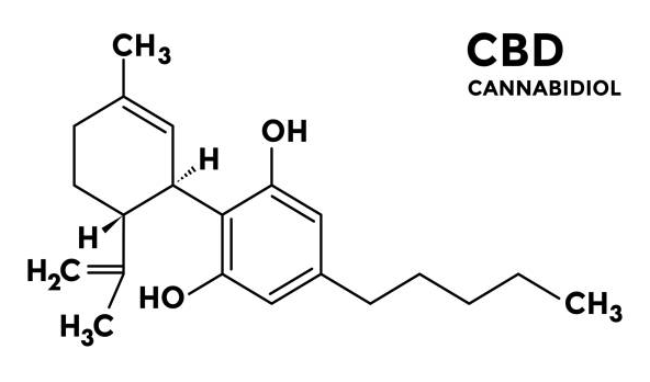
SCCS preliminary assessment on Thiomersal and Phenylmercuric salts as preservatives in cosmetic products.
The SCCS has issued a preliminary safety assessment on thiomersal and phenylmercuric salts – understand the scientific concerns and potential regulatory consequences for cosmetics.