Introduction

In the previous part, it was highlighted that peptides and proteins are interesting cosmetic ingredients, notably because of their anti-aging activities. There is already a plethora of peptide-based cosmetic raw materials on the market, with more being researched constantly.
So far, regulation of these ingredients largely involves their origin (must be synthesized or extracted but not from human cells and other organisms) and marketing claims. Most of the time, these claims are not frivolous but balanced with pronounced physiological activity, often accompanied by side-effects that must be investigated. In this part, the safety aspects of peptides and proteins will be discussed, along with several generalized rules to ensure limited risk for human health when developing or using new peptides and proteins in cosmetic raw materials and products.
Next-Gen vs. Previous-Gen Ingredients
Given their potential bioactivity, peptides and proteins have been the subject of intense medical research over the years. Aspects regarding their properties and safety can therefore be drawn and compared to chemically-defined, small molecules. This is also analogous to cosmetics, since many ingredients used over the decades are chemically-defined small molecules (niacinamide, retinol, etc), compared to the next generation in peptides and proteins (growth factors, collagen subunits, etc).
Table 1: Comparison in profile between small molecules and peptides and proteins
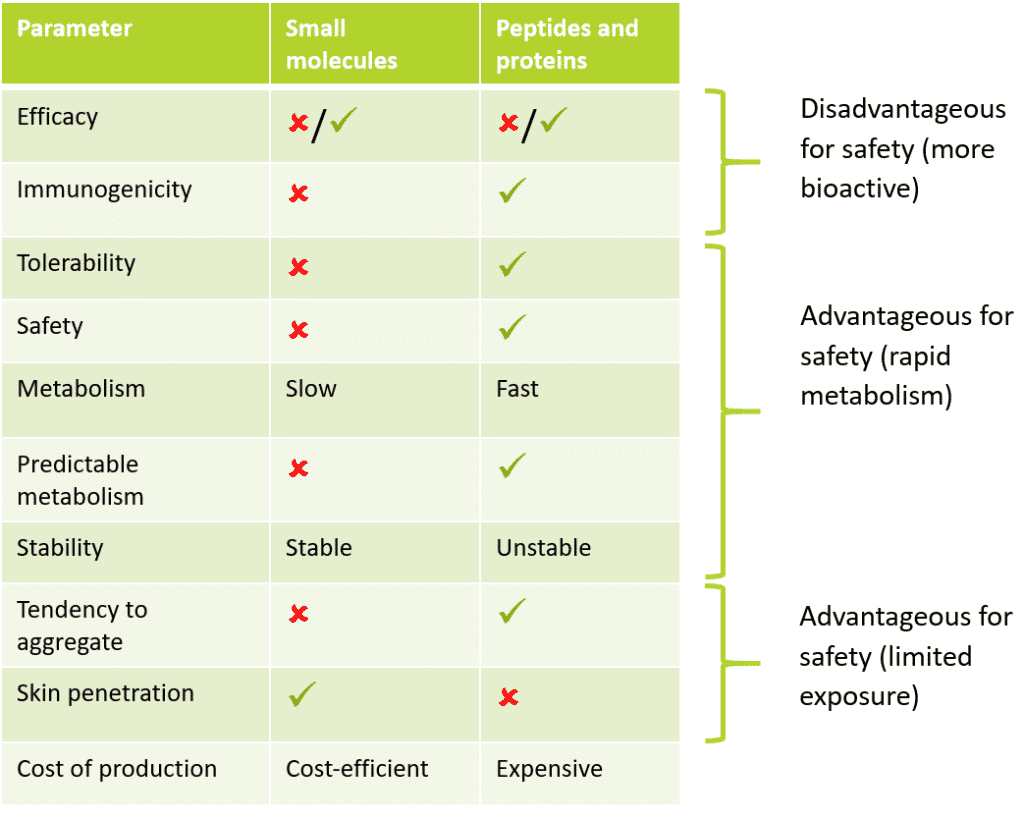
As can be seen above, peptides and proteins have a number of intrinsic properties that are beneficial for an improved safety profile compared to small molecules. For instance, based on pharmaceutical research, peptide and protein drugs were found to have a better safety profile and better tolerated than their traditional small-molecule predecessors. This was due to rapid metabolism and decreased stability leading to decreased bioavailability while also maintaining fewer off-target effects.
In a cosmetics scope, this decreased bioavailability leads to decreased risk to safety. The rationale for this is the central dogma of toxicology: “the dose makes the poison” – Paracelsus 1500’s, considered as the godfather of toxicology. The properties that lend to decreased toxicity associated with peptides and proteins compared to small molecules will be discussed below per toxicological endpoint, many of these endpoints form the basis of toxicological evaluation for any ingredient.
Absorption, Distribution, Metabolism, and Excretion

Absorption, distribution, metabolism, and excretion (or ADME) are the main processes that define exposure to exogenous substances. Considering cosmetics, this is mainly driven by dermal absorption given that cosmetics are normally applied dermally and the skin is actually a significant barrier to exogenous substances.
The skin is made up of various layers, the top-most one being the stratum corneum comprising of tightly packed keratinized cells that serve as a significant barrier to entry of large and/or hydrophilic substances.
Peptides generally have high-to very high molecular weights and they are typically charged at physiological pH which making them hydrophilic, thus contributing to their poor passive skin permeation. In addition, peptides contain multiple amide bonds (as hydrogen bond donor and acceptor groups) which have the potential to affect their diffusion across the skin.
Another aspect deserving consideration is the metabolism and proteolytic activity of the skin is potentially of significance to the transcutaneous delivery of peptides. The skin contains enzyme systems comparable with those found in other tissues for example the liver. Endogenous enzymes such as deaminases, esterases, and aminopeptidases are found in all compartments of skin and may contribute to the rapid metabolism of peptides and proteins before entering systemic circulation assuming these peptides and proteins were even able to cross the stratum corneum initially.
As such, the skin serves as a significant barrier to peptides and proteins based on the size of these molecules and the metabolic capacity of the skin, leading to limited systemic exposure. An example best showing this is perhaps of that of pentapeptide-4 (KTTKS) which despite being a peptide of only 5 amino acids, was found to not be present in receptor fluid or any of the skin layers (the stratum corneum, epidermis, and dermis) in hairless mouse skin (Choi et al., 2014).
Phototoxicity

Potential for phototoxicity can be identified based on in silico parameters. For one, the extinction coefficient – the ability of a substance to absorb light – can be used as an indication of potential risk. For instance, substances with extinction coefficients of less than 1 000 L mol-1 cm-1 are deemed less of a phototoxic risk since this low level of light absorbance is unlikely to prove harmful (Henry et al., 2009). This opinion is also shared by the IHC (International Conference on Harmonisation, 2013) and the European Medicine Agency (European Medicines Agency, 2011).
Specifically for peptides and proteins, there are a number of web-based tools able to predict extinction coefficients based on the presence of tyrosine, tryptophan, and cysteine residues, amino acids known to absorb light. As such, limiting tyrosine, tryptophan, and cysteine residues can radically limit the protein or peptide’s extinction coefficient and therefore limit risk for phototoxicity.
Genotoxicity

In general, peptides and proteins are not expected to be potentially genotoxic. In one of its guidelines (ICH S6(R1)), the ICH (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use) states that “It is not expected that these substances (peptides/proteins) would interact directly with DNA or other chromosomal material” (International Conference on Harmonisation, 2011). This was further confirmed by other studies where 78 proteins were tested for genotoxicity with only four appearing to elicit reproducible genotoxic effects.
The activity for one of these peptides was attributed to its linker molecule, while no clear rationale could be established for the remaining 3 peptides except for potential enzymatic/hormonal activity. In fact, it was concluded that for the ‘average’ protein, electrophilic reactions are difficult to envision (Gocke et al., 1999).
Nevertheless, this shows that significant attention should be paid to the production processes used to produce these peptides and proteins since some reagents may be genotoxic, while purification steps need to be incorporated in the production processes to limit the carry-over of potential by-products to the raw material.
Sensitisation

The most significant type of sensitisation for cosmetic products is contact dermatitis which is classified as type 4 sensitisation and involves mostly T-cells. It is generally accepted that skin sensitizers are electrophilic and able to react with nucleophilic groups on proteins such as cysteine and lysine units.
Another important mechanism is based on free radical reactions (Roberts et al., 2012). Since both types of reactions are unlikely with the peptide under investigation and because the absorption of such compound is expected to be low, the risk of skin sensitization is considered as low.
However, given that many proteins and peptides present in food also serve as allergens, proteins and peptides as used in cosmetics deserved to be assessed for this type of allergenicity. Specifically, immediate hypersensitivity such as allergy and anaphylaxis, involves different mechanisms compared to contact dermatitis.
In type 1 sensitisation, the effector cells are mostly antibody-producing cells such as mast cells and IgE antibodies. However, from the scientific opinions of the SCCS and CIR on hydrolysed wheat proteins, it becomes clear that peptides with a molecular weight below 3.5 kDa have a low sensitization capacity (SCCS, 2014). As such, for a type 1 sensitization reaction to occur an allergen must bind two IgE antibodies. Therefore, an allergen must contain at least two IgE binding sites each with a minimum of 15 amino acids long. This implies that the minimal size for protein allergens is approximately 30 amino acids (Huby, 2000).
Potential Hazard to Human Health

Based on what was discussed above, one might assume that peptides and proteins do not have significant toxicological profiles, which would be incorrect. In fact, peptides and proteins may harbour hazard to human health that is driven by bioavailability and exaggerated pharmacology. For instance, proteins in the form of receptors and enzymes are considered as the functional unit that facilitates normal bodily functions. The same is true for peptides which include signalling molecules. As such, if an imbalance in homeostasis were to occur through increased or decreased activity, adverse effects could arise. However, identifying such “exaggerated pharmacological” events are not easily identified in current safety testing approaches.
To identify potential hazard to human health, and given the nature of proteins and peptides in that, a cascade of biological events could occur that could lead to an adverse effect, a holistic approach would be needed. For this, it could be envisioned that next-generation omics technologies would help greatly. Briefly, these technologies allow a comprehensive overview of molecular events occurring at a gene and protein level. Once a network of affected genes and/or proteins are identified, this can then be tied to molecular modes of action know to give rise to adverse health effects. This is in line with the Adverse Outcomes Pathway (AOP) approach where apical adverse health effects are predicted from molecular initiating events, an approach greatly gaining traction in regulatory toxicology. Successful implementation of this would require developing skills in these fields, while ensuring that testing is not cost-prohibitive. Furthermore, some AOPs have been developed and validated while others are at various stages of acceptance and development. In fact, there is still a significant amount of work needed to map toxicological molecular pathways as AOPs. As such, given that hazard to human health cannot readily be identified using these approaches yet, uncertainty is managed by ensuring the limited bioavailability of these cosmetic ingredients.
Conclusion

As is described above, peptides and proteins benefit from a number of properties that could limit health risk. These properties are especially important in light of the animal testing ban for cosmetic ingredients. Nevertheless, significant modifications to these ingredients should be avoided to maintain these characteristics. These briefly include:
- No extensive modification to significantly increase absorption – this serves to maintain the central dogma of toxicology; limited exposure = limited risk
- Limited modifications to amino acids – natural amino acids are not expected to be genotoxic, carcinogenic, nor reproductive toxicants while being rapidly metabolised
- Limit amount of tyrosine, tryptophan, and cysteine residues – limit the potential for phototoxicity
- Include purification steps in production processes – to limit carry-over of potentially dangerous impurities
These considerations are important in light of the ban on animal testing for cosmetic ingredients where toxicologists have to rely on data and tools other than traditional animal toxicological tests. Furthermore, as mentioned in Part I, cosmetics and drugs are distinguished based on their intended uses which are primarily driven by their claims. However, based on the Working Group on Cosmetic Products manual on borderline products, substances which restore, correct, or modify physiological functions by exerting pharmacological activities are identified by “virtue of its presentation” (so claims as described above), or “by virtue of its function”. The latter is “assessed by considering all characteristics of the product including absorption, concentration, route of administration, frequency of application, application site, and the degree of penetration”. As such, it becomes obvious the role of understanding how peptide and protein characteristics intertwine to drive toxicological profiles in determining when an ingredient is a drug or cosmetic.
References
Choi, Y.L., Park, E.J., Kim, E., Na, D.H., and Shin, Y.H. (2014). Dermal stability and in vitro skin permeation of collagen pentapeptides (KTTKS and palmitoyl-KTTKS). Biomol. Ther. 22, 321–327.
European Medicines Agency (2011). Questions and answers on the ‘ Note for guidance of photosafety testing.’
Gocke, E., Albertini, S., Brendler-Schwaab, S., Müller, L., Suter, W., and Würgler, F.E. (1999). Genotoxicity testing of biotechnology-derived products: Report of a GUM task force. Mutat. Res. – Rev. Mutat. Res. 436, 137–156.
Henry, B., Foti, C., and Alsante, K. (2009). Can light absorption and photostability data be used to assess the photosafety risks in patients for a new drug molecule? J. Photochem. Photobiol. B Biol. 96, 57–62.
Huby, R.D.J. (2000). Why Are Some Proteins Allergens? Toxicol. Sci. 55, 235–246.
International Conference on Harmonisation (2011). Preclinical safety evaluation of biotechnology-derived pharmaceuticals.
International Conference on Harmonisation (2013). Photosafety evaluation of pharmaceuticals.
Roberts, D.W., Basketter, D., Kimber, I., White, J., McFadden, J., and White, I.R. (2012). Sodium metabisulfite as a contact allergen–an example of a rare chemical mechanism for protein modification. Contact Dermatitis 66, 123–127. SCCS (2014). Opinion on hydrolysed wheat proteins.
This article was written by Dr. Boris Krivoshiev, former Principal Toxicologist, during his time at BIORIUS.


