Retinol and Retinyl Esters – A New Approach to Manage these Ingredients
May 16th, 2017
Retinol and its esters (Retinyl Acetate, Retinyl Propionate and Retinyl Palmitate) are active ingredients commonly found in anti-aging skincare products.
As communicated below, Retinol and the three outlined above esters have been evaluated by the SCCS (Scientific Committee on Consumer Safety). The Scientific Opinion, finalized in October 2016, concluded that Retinol (in combination with its esters) is safe when used at maximum 0.3 % in face creams, hand creams and rinse-off products. A maximum concentration of 0.05 % was deemed safe when used in body lotions.
BIORIUS carefully evaluated this Scientific Opinion and tends to disagree with its conclusions, regarded as overly conservative. Although the law requires BIORIUS to consider SCCS’ Opinions in its evaluations, these scientific dossiers are not pieces of legislation and can be implemented with some flexibility. These ingredients will not get regulated before 2 or 3 years. In the meantime, BIORIUS plans to adopt a specific strategy. Here is an illustration of this strategy for a face cream:
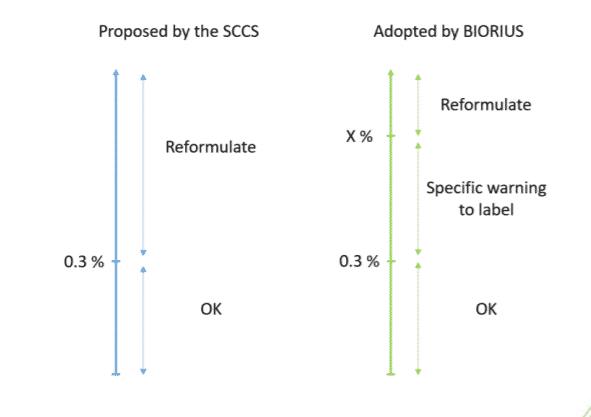
“X” represents the maximum concentration of the sum of Retinol and its esters that can be safely used in a cosmetic product. This percentage varies depending on the product category. For an accurate calculation of this percentage, you can open this Excel spreadsheet and follow the instructions. For instance, X = 1.25 % if only Retinol is used in the face cream.
When the percentage of Retinol and its esters is above the concentration of 0.3% and below the maximum safe level (X), the specific warning to label “Do not use this cosmetic product with other retinol-containing cosmetic products during your treatment.” will have to be added. This sentence would be less worrying than the current warning “Avoid during pregnancy”. For products already notified, we recommend that the previous warning be replaced with the new wording.
This solution is the best solution that our toxicologists found to mitigate the consequences of this SCCS Opinion. However, it is noteworthy that this solution is temporary and the restrictions will have to be strictly applied if the SCCS Opinion is transposed into the legislation.
We encourage you to get familiar with this Excel spreadsheet and to come back to us in case you have questions about this new risk assessment/risk management method.
Any questions?
Publication of the Draft SCCS Opinion on Retinol and Retinyl Esters
May 6th, 2016
As mentioned in this article (10), Retinol and Retinyl Esters are currently on the table of the SCCS, which is in charge to evaluate its safe use in cosmetic products. Mandated by the EU Commission, the SCCS was only allowed to answer the questions asked, the main one being:
On the basis of data provided does the Scientific Committee on Consumer Safety (SCCS) consider Vitamin A (retinol, retinyl palmitate, retinyl acetate, retinyl linoleate and retinal) safe when used as cosmetic ingredient:
a) in body lotions up to the maximum concentration of 0.05 % of retinol equivalent?
b) in hand/face cream, leave-on (other than body lotions) and rinse-off products up to the concentration of 0.3 % of retinol equivalent?
In its Scientific Opinion, the SCCS concluded that 0.05% of retinol equivalent in body lotions and 0.3% in hand/face cream, leave-on (other than body lotions) and rinse-off products are safe use levels.
The good news is that the SCCS did not request to lower these use levels. The bad news is that the EU Commission will probably use these rather low concentrations of 0.05% and 0.3% as the basis for developing their risk management measure. Unless the Industry voicesits concerns, a new entry will be created in Annex III of the EU Cosmetics Regulation to limit the use of retinol and retinyl esters to these concentrations.
This Scientific Opinion is still a draft until June 21st , 2016 and you may want to participate in the public consultation to provide safety data that may change the conclusions of this risk assessment.
If so, please, let us know at your earliest convenience. Biorius will collect the comments and submit them to the EU Commission.


