In this article we will delve into the details of regulations in Australia and Europe for cosmetic products.
First, we will discuss the requirements for selling cosmetics in Australia, followed by the requirements for selling in Europe.
Compliance Requirements in Australia: What You Need to Know
As outlined in our page on regulatory services provided by Biorius for the Australian market, the Australian Industrial Chemicals Introduction Scheme (AICIS) oversees the regulation of chemicals, while the Australian Competition and Consumer Commission (ACCC) regulates label requirements, including the International Nomenclature of Cosmetic Ingredients (INCI) list.
To put it simply:
- The cosmetic product formula must meet the requirements set by AICIS and the Therapeutic Goods Administration (TGA).
- The labels must comply with the requirements set by the ACCC.
Regarding ingredient registration in Australia, it involves studying the human and environmental toxicity of the substances. Factors such as the application, amount introduced, concentration, and more must be taken into account. Based on this categorization, a risk category is assigned.
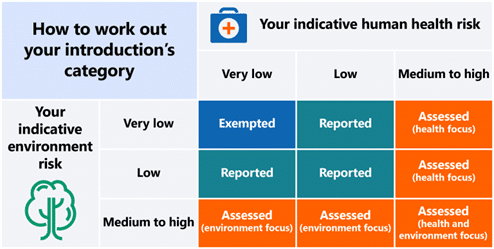
If a substance falls into the “Assessed” category, a “Safety Assessment” (which is quite rare) must be prepared. Biorius can assist with the categorization and Safety Assessment work.
In order to sell cosmetic products in Australia, cosmetic companies must have an importer based in Australia who is registered with AICIS. This importer is responsible for ensuring compliance of the distributed products. If the company sells from abroad (e.g., via e-commerce), there are two options:
- The company can register with AICIS as a foreign company and thus become an importer.
- Alternatively, the company can use an agent based in Australia to perform the procedure on its behalf.
In either case, the importer is responsible for ensuring compliance of the distributed products.
Biorius can assist in ensuring product compliance by:
- Conducting an evaluation study to verify whether the formula complies with Australian requirements.
- Providing recommendations if additional data is needed to achieve compliance.
- Offering information regarding AICIS registration as a foreign company.
Compliance Requirements in EU: Compliance for Australian Cosmetics (and more) in Europe
As mentioned in our article How to gain EU compliance in 3 steps a cosmetic sold in the EU and UK must comply with the following requirements:
- A PIF (Product Information File) containing the CPSR (Cosmetic Product Safety Report) signed by a Safety Assessor;
- An EU CPNP & UK SCPN registration number;
- A Responsible Person for the EU and UK.
In Australia, there is no requirement for a PIF or notification to portals. However, it is important to remember that a cosmetic product intended for sale in Australia must comply with the rules set by AICIS, TGA, and ACCC. The EU/UK Responsible Person can be equated with the importer based in Australia, who is registered with AICIS and also responsible for ensuring compliance and safety of the imported cosmetic products.
Regulatory Services offered by Biorius
Biorius can assist you with regulatory compliance. Here are the regulatory services we provide for the Australian market:

Regulatory Service Australia
The regulatory service dedicated to the Australian market includes:
- Formula review: Evaluation of the formula according to the requirements set by AICIS and TGA.
First, our experts work on categorization of the product to make sure it can be considered a cosmetic.
Then, they proceed to a toxicological analysis to check the safety of the formula.
Finally, they check the ingredients against various standards:
- AIIC (Australian Inventory of Industrial Chemicals) (including the confidential part of the inventory).
- Customs regulations on prohibited imports.
- SUSMP (Standard for the Uniform Scheduling of Medicines and Poisons).
Upon completion of this first stage, the client receives a detailed report on each stage of the formula review (product categorization, toxicological analysis, and ingredient review).
- Label and claims review: Evaluation of the label according to the requirements established by the ACCC.
Evaluation of product label (ingredient list, symbols, legal requirements, …) and substantiation of claims.
Strategic recommendations are provided to update the label.
A table with the following elements is provided to the client:
- Mandatory elements
- Presence of mandatory elements (primary packaging, secondary packaging and package insert)
- Expert comments – Corrective actions to be taken
- Final INCI list
The file provided to the client also contains a part on indications with the following information:
- Indications
- Conclusion on each indication
- Expert comments – Corrective actions to be taken
EU/UK Regulatory Services
While regarding the regulatory service for EU and UK, we offer a turnkey solution:
- Formula Review – divided into Composition Review and Product Compliance Review;
- CPSR Cosmetic Product Safety Report (A&B);
- Label and claims review;
- PIF preparation and notification to CPNP and SCPN portal;
- Legal Representation in EU/UK.
Contact us!
For more information about the regulations in Europe and Australia, please feel free to visit our website at the dedicated page and contact us at info@biorius.com.


