Comparison between the US and EU cosmetics regulations
Navigating cosmetics regulations can be a daunting task, especially when comparing the stringent requirements of the European Union (EU) with the relatively flexible framework of the United States (US). Here, we break down the major differences, offering insights into how businesses can successfully bring their products to both markets.
The Fundamental Difference: Spirit of the Law
- EU: Known as a protective society, the EU prioritizes safety and certainty. Its regulatory framework is highly directive, leaving minimal flexibility for manufacturers. With numerous restrictions and prohibitions, the focus is on ensuring that products are completely safe before they reach consumers.
- US: As a litigious society, the US emphasizes freedom and responsibility. While products must be safe for consumers, manufacturers are granted more freedom to demonstrate safety through toxicological evaluations. This approach allows some products to be sold in the US that would not meet EU standards. However, the US has stricter regulations regarding dyes.
European Cosmetics Regulations: Rigorous and Comprehensive
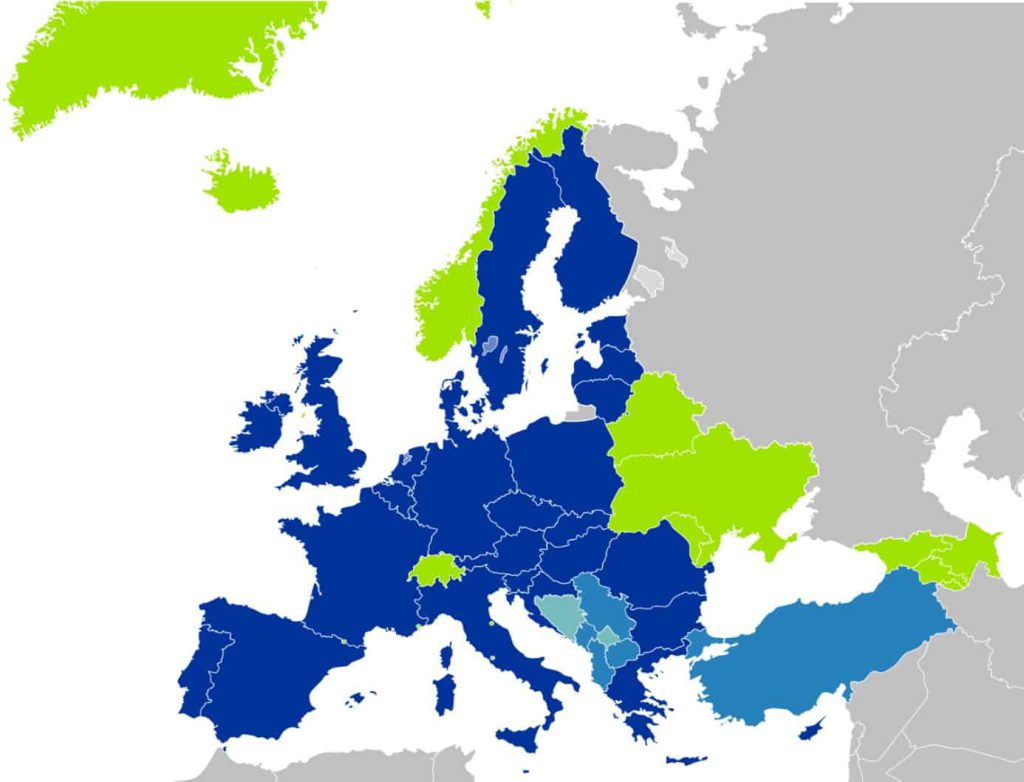
Dark blue countries: in all these countries, the European Cosmetics Regulation applies at 100%
Austria, Belgium, Bulgaria, Croatia, Cyprus, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Malta, Netherlands, Poland, Portugal, Romania, Slovakia, Slovenia, Spain, Sweden.
Light blue + Green countries: the European Cosmetics Regulation applies with some specificities
Albania, Armenia, Azerbaijan, Belarus, Bosnia and Herzegovina, Georgia, Iceland, Kosovo, Macedonia, Moldova, Montenegro, Norway, Serbia, Switzerland, Turkey, Ukraine
With Brexit, the UK has become a light blue or green country.
More information on this matter here.
Before selling cosmetic products in Europe, you must ensure they meet strict safety and compliance standards to protect consumers.
The EU’s Cosmetic Regulation applies across all Member States, with specific adaptations in countries like the UK post-Brexit. It’s recognized as the most complex and detailed regulation globally.
You are required to demonstrate that your products will not harm consumers. Each product must fully comply with the EU Cosmetic Regulation, ensuring it meets all legal and safety requirements. Additionally, you must appoint an EU Responsible Person with an office in Europe to oversee compliance and act as the point of contact for regulatory authorities.
Compliance is non-negotiable—be prepared to meet these obligations before entering the market:
Conduct Extensive Reviews:
- Formula Review: Verify compliance with restricted ingredients.
- Laboratory Testing: Depending on the product formula. Examples include stability and compatibility tests, microbiological testing. More here.
- CPSR A & B (Cosmetic Product Safety Report): extensive assessment of the product completed and signed by an accredited expert or toxicologist.
- Label and Claims Review: Ensure accurate and compliant product labeling with mandatory information.
Prepare Regulatory Documentation:
- PIF (Product Information File): A complete file containing formula details, CPSR, and labeling.
- EU CPNP Notification: Register products in the EU’s Cosmetic Products Notification Portal to announce their sale in Europe.
Appoint an EU Responsible Person:
This person ensures compliance and serves as the primary contact for authorities and consumers in case of issues. They also bear responsibility for non-compliance penalties.
The role of the Responsible Person carries significant responsibilities. They are accountable for ensuring compliance with EU regulations and will be held liable in cases of non-compliance, including paying any fines resulting from regulatory breaches.
EU Overview:
In the European Union, regulations are directly applicable in all Member States without the need for transposition into national law. However, individual countries may implement additional provisions for areas not covered by the Cosmetics Regulation, such as environmental concerns or specific national legislations (e.g., in France or Germany).
More information on the EU Cosmetics Regulation available here.
US Cosmetics Regulations: Flexible but Evolving
Important Update:
On December 29, 2022, the most significant amendment to U.S. cosmetic regulations (21 U.S.C., Sec. 321-392) since 1938 was enacted: the now well-known MoCRA (Modernization of Cosmetic Regulation Act).
This legislation introduces substantial new obligations for the cosmetics industry and is expected to have a major impact on brands distributing products in the USA. Beyond its new regulatory provisions, MoCRA represents a paradigm shift in the oversight of cosmetic products in the region.
Key highlights of MoCRA include:
- Increased FDA Powers: Enhanced surveillance, inspection, and injunction capabilities to protect public health.
- Enforcement Authority: The FDA can now suspend facility registrations and mandate product recalls.
With these changes, the regulation of cosmetics in the USA is moving closer to the philosophy of European law.
Please Note: The information in the original article may no longer be entirely up to date due to these significant developments.
-
The FDA Federal Food, Drug, and Cosmetic Act is a very short law (1 page). -
The related Regulation (CFR, Title 21, Chapter 1, Subchapter G, Sections 700-740) is short too. -
Most of the States only apply the Federal legislation but it exists national provisions (example, California legislation)
From a European perspective, U.S. cosmetics regulations are relatively straightforward and quick to navigate.
However, it’s crucial to ensure that all requirements are met to avoid potentially lengthy, costly, and stressful legal disputes—something every brand would prefer to avoid.
In practical terms, this means ensuring your products are compliant and prepared for inspection by U.S. authorities. During an inspection, you may be asked to provide documentation such as your formula review, label and claims review, and toxicological risk assessment.
It’s important to note that approval for import into the U.S. does not necessarily mean your products comply with U.S. cosmetic regulations.
Simpler Compliance Requirements:
- Formula Review
- Toxicological Risk Assessment (TRA)
- Label and Claims Review
Voluntary Cosmetic Registration Program (VCRP):
While not mandatory, this program provides additional protection by offering the FDA easier access to product information during inspections.
State-Level Regulations:
The primary challenge in U.S. cosmetics regulation often arises from state-specific requirements. Among the states, California stands out as the most complex due to its additional regulations, including the California Air Resources Board, the California Safe Cosmetics Program, and Proposition 65.
To simplify this process, Biorius offers tailored solutions:
- A U.S. Federal Regulatory Package, covering federal-level compliance.
- A U.S. Federal and States Regulatory Package, ensuring compliance with both federal and state-specific regulations across the entire country.
For more detailed information about U.S. cosmetics regulations, click here.
| Comparison | EU | US |
|---|---|---|
| Regulated Substances | Yes (more than 2,000) | No (only 11 substances) |
| PIF (Product Information File) | Yes | No |
| Labeling Elements | Rules clearly established | Differences with the EU |
| Marketing Claims | Specific regulation | Very limited guidance |
| Notification | Centralized & electronic (on CPNP) | No (voluntary registration possible) |
| Responsible Person (RP) | One Responsible Person | All stakeholders are responsible |
| Animal Testing | Prohibited | Accepted for now (projects ongoing) |
| Nanomaterials | Severely regulated | Unregulated |
| Good Manufacturing Practices (GMP) | GMP compliance required | GMP compliance required |
Labeling Rules: EU vs. US
Labeling standards differ significantly between the two regions. Here are a few notable contrasts:
- Business Information: EU requires manufacturers, packers, or distributors, while the US allows flexibility in listing.
- Warnings: Specific warnings are mandatory in the EU for aerosols, foaming products, and tanning products.
- Net Content: The EU uses the metric system, while the US allows US customary units with metric as an option.
- Ingredient Listing:
-
- The EU mandates disclosure of fragrance allergens and nanomaterials.
- The US does not require these but allows trade secrets to be listed as “and other ingredients.”
Claims: What Can Be Said?
Both the EU and US prohibit claims that exceed the scope of the definition of a cosmetic product. However:
- The EU enforces stricter guidelines (e.g., EU Regulation No. 655/2013) requiring clear substantiation.
- The US requires claims to be truthful and non-misleading but is less prescriptive about substantiation.
Recommendations for Global Compliance
To streamline compliance and reduce risk, brands should consider:
- Formulating products to meet EU standards, which often cover US requirements.
- Working with regulatory professionals to craft a single product label suitable for both regions.
How Biorius Can Help
Understanding and adhering to cosmetics regulations is crucial for global success. At Biorius, we specialize in simplifying this process, offering tailored solutions for compliance in both the EU and US markets. From regulatory documentation to labeling and claims reviews, we ensure your products meet the highest standards, giving you the confidence to expand your brand worldwide.
For more information on EU and US cosmetics regulations, feel free to contact us.
Author

Christophe Brault-Chevalier
