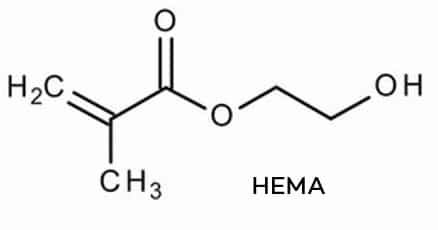
Hydroxyethylmethacrylate (HEMA) and Di-HEMA Trimethylhexyl Dicarbamate (Di-HEMA TMHDC) are two colorless viscous liquids that readily polymerize when exposed to UV-light. Because of their physical properties, these monomers are typically used in nail enhancement products. The skin sensitization potential of these cosmetic ingredients raised concerns since 2016.
The restriction of HEMA and Di-HEMA TMHDC to professional nail products only has been adopted. The related regulation, the Commission Regulation (EU) 2020/1682, has been published on November 12th, 2020.
The use of HEMA and Di-HEMA TMHDC is restricted as follows:
- Only to be used in professional nail products. Any other use is prohibited.
- Warnings to be added to the product labels:
- “For professional use only”
- “Can cause an allergic reaction”
Transition periods are granted as below:
- From June 3rd, 2021 products containing that substance and not complying with those conditions shall not be placed on the Union market.
- From September 3rd, 2021 products containing that substance and not complying with those conditions shall not be made available on the Union market.
We hope that this letter will be of help and stay at your disposal for any questions related to this topic.
Best regards,
Ms. Stéphanie Annet
EU Regulatory Supervisor