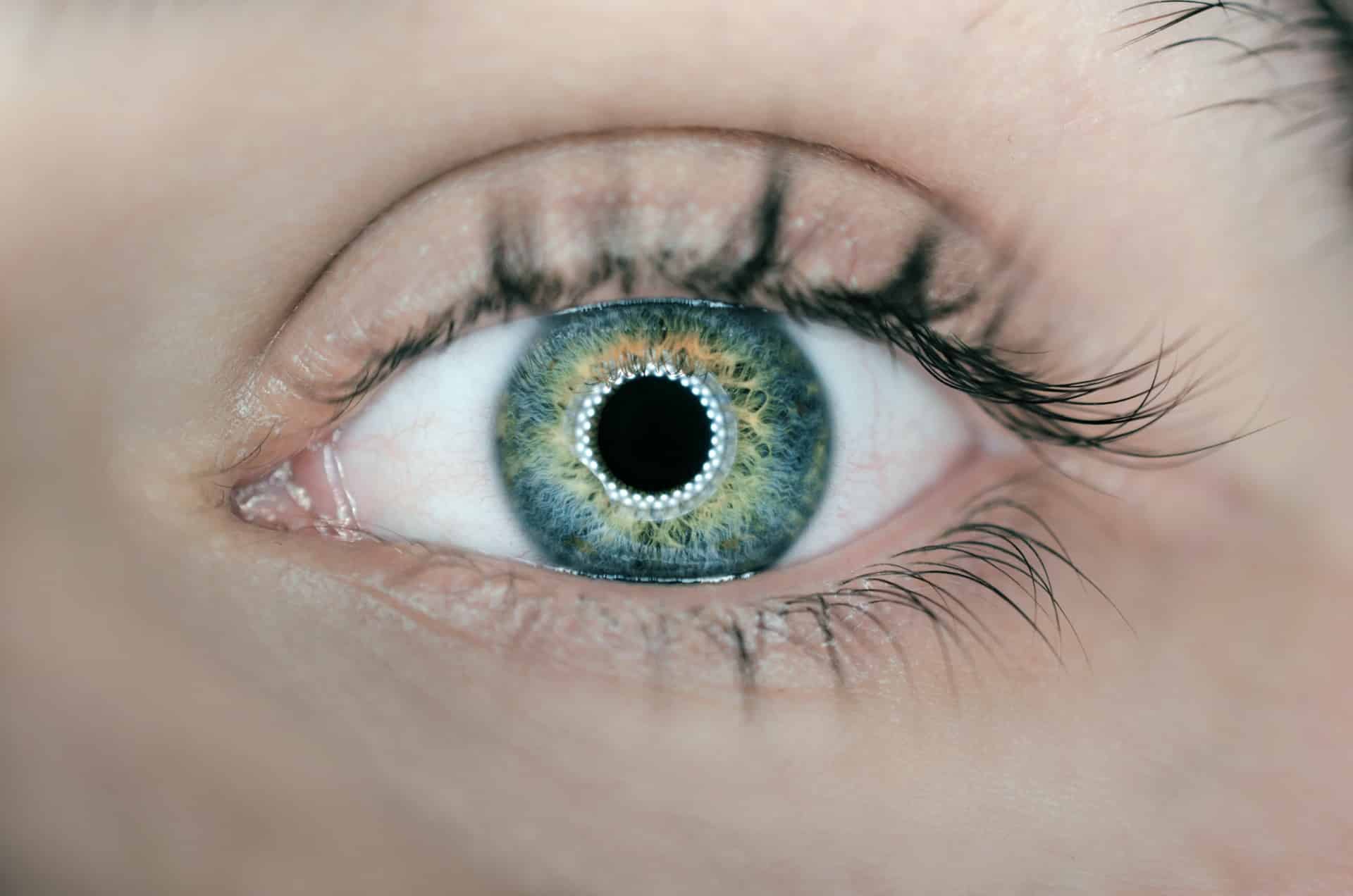
The Borderline Between a Cosmetic Product and a Medical Device
One important change in the Medical Device Regulation (EU) 2017/745 (the MDR) is the integration of 6 groups of products in its scope: the “Cosmetic Products” listed in Annex XVI which reads the following:
LIST OF GROUPS OF PRODUCTS WITHOUT AN INTENDED MEDICAL PURPOSE REFERRED TO IN ARTICLE 1(2)
- Contact lenses or other items intended to be introduced into or onto the eye.
- Products intended to be totally or partially introduced into the human body through surgically invasive means for the purpose of modifying the anatomy or fixation of body parts with the exception of tattooing products and piercings.
- Substances, combinations of substances, or items intended to be used for facial or other dermal or mucous membrane filling by subcutaneous, submucous or intradermal injection or other introduction, excluding those for tattooing.
- Equipment intended to be used to reduce, remove or destroy adipose tissue, such as equipment for liposuction, lipolysis or lipoplasty.
- High intensity electromagnetic radiation (e.g. infra-red, visible light and ultra- violet) emitting equipment intended for use on the human body, including coherent and non-coherent sources, monochromatic and broad spectrum, such as lasers and intense pulsed light equipment, for skin resurfacing, tattoo or hair removal or other skin treatment.
- Equipment intended for brain stimulation that apply electrical currents or magnetic or electromagnetic fields that penetrate the cranium to modify neuronal activity in the brain.
While some products listed are easy to identify, such as “decorative” contact lenses, the verbiage used makes it somehow difficult to understand what devices are concerned. We’ll first try to clarify this point.
THE GROUPS OF PRODUCTS WITHOUT AN INTENDED MEDICAL PURPOSE REFERRED TO IN ARTICLE 1(2)
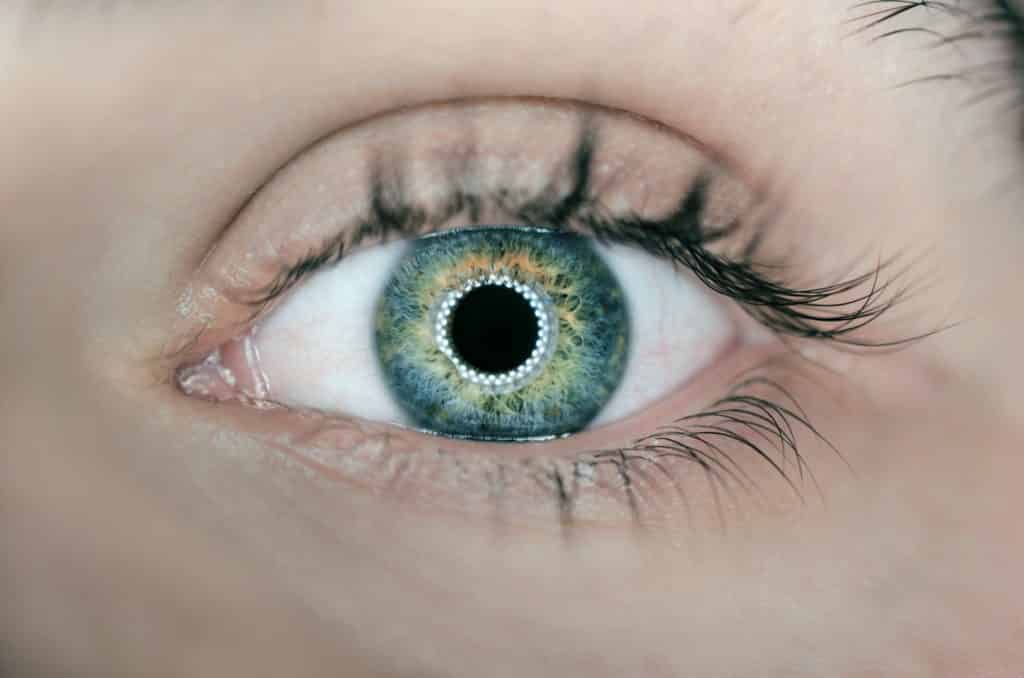
Contact lenses or other items intended to be introduced into or onto the eye.
These devices are ‘decorative’ lenses to change the color of your eyes for example from brown to blue or eyeball piercings as shown in the picture.
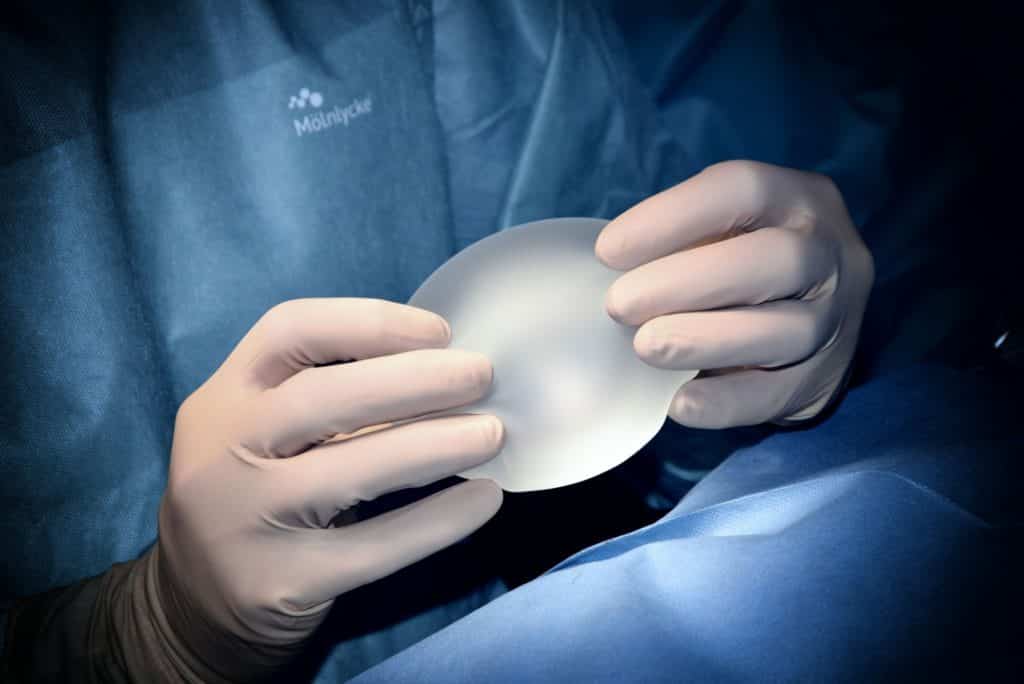
Products intended to be totally or partially introduced into the human body through surgically invasive means for the purpose of modifying the anatomy or fixation of body parts with the exception of tattooing products and piercings.
These devices are breast implants used for female breast augmentation but also all kinds of implants for males for bicep, calf, or pectoral implants in addition to other implants in the face to modify the appearance.
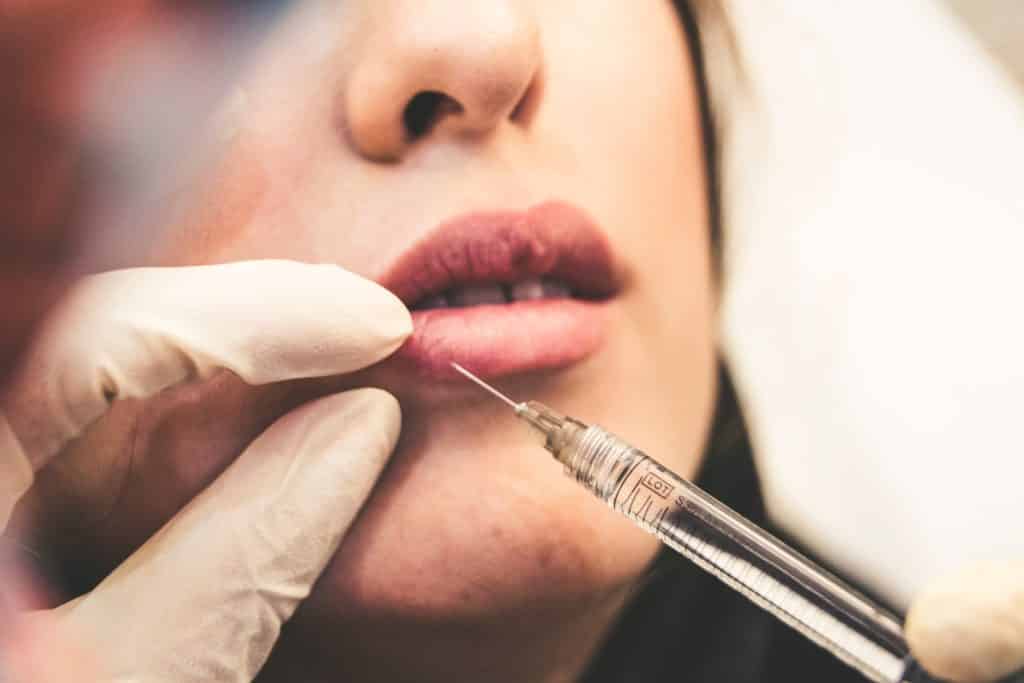
Substances, combinations of substances, or items intended to be used for facial or other dermal or mucous membrane filling by subcutaneous, submucous or intradermal injection or other introduction, excluding those for tattooing.
These devices are known as “dermal fillers” and are injectable implants commonly used in aesthetic clinics to remove wrinkles, plump lips and other areas of the face but may also be used in the genital area. A famous ingredient is Hyaluronic Acid. But what are those substances intended for the face and the derma by “other introduction” means? Are mesotherapy products the targeted products? If it is so, there is a lot to be done in this practice.
Equipment intended to be used to reduce, remove, or destroy adipose tissue, such as equipment for liposuction, lipolysis or lipoplasty
These are devices used for liposuction but also those using cryothermy or other technics.
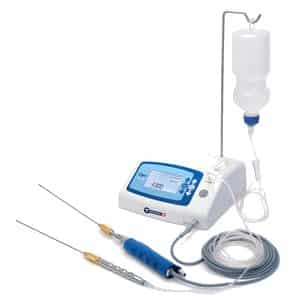
High intensity electromagnetic radiation (e.g. infra-red, visible light and ultra- violet) emitting equipment intended for use on the human body, including coherent and non-coherent sources, monochromatic and broad spectrum, such as lasers and intense pulsed light equipment, for skin resurfacing, tattoo or hair removal or other skin treatment.
These devices use light to have an effect on hairs or skin. One of the difficulties here is the lack of detailed definition for such devices for example what is the level of the light radiation to be considered as high intensity?

Equipment intended for brain stimulation that apply electrical currents or magnetic or electromagnetic fields that penetrate the cranium to modify neuronal activity in the brain.

These devices are intended to treat depression, relieve pain or without any medical purpose stimulate the brain capacity. They may use electrical current, magnetic, or electromagnetic fields to do so.
Aesthetic or Medical Purposes? What does the New Regulation say?
In fact, some of these devices without medical purposes may commonly be CE marked and their manufacturers are already familiar to Notified Body conformity assessments. The same goes for breast implants, dermal fillers, and IPL equipment. For these devices, there are both medical purpose applications and non-medical ones. The clarification brought by annex XVI was needed because the Directives were applied differently in some EU countries and devices considered as Medical Devices were not in this category in other countries. The uniform application of the new Regulation for all EU Member States is making the same rules applicable everywhere in the European Union.
In addition, this change will put an end to well-known hypocrisy which pushes manufacturers to twist their intended purposes pretending that there was a medical indication when most of their devices were purely for aesthetic purposes. For example, breast implants were indicated for breast implant reconstruction after mastectomy due to cancer but a lot of them were sold for breast augmentation. Dermal filler manufacturers had to find patients with very specific diseases when their devices were used mainly for women in a specific age range. Manufacturers will no longer have to present clinical benefits and may limit their demonstration to clinical safety.
For decorative contact lenses, it’s another story since their manufacturers were not previously concerned by the medical device regulations. They will have to demonstrate their device’s safety, to implement a Quality Management System and to dramatically change their documentation. In fact, they will have to comply with the regulation the same way as corrective contact lens manufacturers excluding the clinical benefit demonstration which will be limited to the safety part. The standards applicable to each group of devices will be the ones existing for similar technology: for example, the standards concerning corrective contact lenses will be applicable to decorative contact lenses.
When will these new rules become applicable?
The original plan was to publish Common Specifications before 26 May 2021 the Regulation Date of Application, and to give a 6 month delay to manufacturers to be compliant. But to date (December 2021), there have been no Common Specification published on this topic and with the lack of notified body resources it may not be a high priority matter for the European Commission.
Is “wait and see” an option? Probably not as getting ready for this Regulation could take from 12 to 36 months depending on where you start from, what resource you can spend on it and the chosen notified body’s availability. The 6 months period planned in the regulation to prepare for it being largely underestimated.
So, it’s time to contact Biorius to prepare your products and company for this major regulatory change.