What is the difference between Shelf Life, PAO and Expiration Date?
In the era of “Conscious beauty”, the shelf life and expiration date of cosmetics is an important issue for many brands. Here is a comprehensive compliance guide on this topic.
EU context: PAO VS Cosmetics expiration date
According to the EU Cosmetic Regulation, art. 19 (c), it is stated that “Indication of the date of minimum durability shall not be mandatory for cosmetic products with a minimum durability of more than 30 months. For such products, there shall be an indication of the period of time after opening for which the product is safe and can be used without any harm to the consumer”
Table of Content
From this article, we notice that there are two options to mention an expiry date on the label:
- The expiration date: this is the limit date by which the product is still valid, used or not by the consumer.
- PAO: this is the Period After Opening, that in practice means how long people can use the cosmetic product after opening and using it for the first time.
Two scenarios…
Therefore, there are two types of scenarios.
- The product’s shelf life is equal to or more than 30 months therefore the product is eligible for a PAO instead of an expiration date. This doesn’t mean the PAO is 30 months (shelf life and PAO are two different things) but that the PAO (in months) must then be calculated based on other parameters. During the safety assessment and registration process of a “classic” cosmetic product, Biorius does this calculation and gives you the PAO period, taking into account: Stability and Challenge tests results (read more on laboratory testing), frequency of use, exposure area, water content …
- If the product’s minimum durability is below 30 months, in this case PAO is no longer acceptable and you must display the date of minimum durability. If the stability is severely in doubt and depending on the reasons, the product might need to be reformulated.
…and some exceptions
It must be borne in mind that for some products the PAO is not relevant. For example single use products, products where the packaging does not allow physical opening of the products…
And on the label?
Again, there are two scenarios:
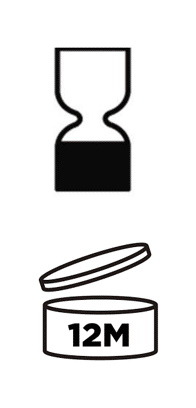
- If the date of minimum durability is applicable, you must use the “hourglass symbol” reported in the Annex VII of the Cosmetic Regulation. Or you could also use the words “‘best used before the end of’”, but bear in mind that the sentence must be translated in the language of the country where the product will be marketed.
- Instead, if the PAO is applicable, you must show the open-jar symbol (reported in the same Annex VII) followed by (or containing) the period in months:
Cosmetics expiration date: What about the US?
According to the FDA, “there are no U.S. laws or regulations that require cosmetics to have specific shelf lives or have expiration dates on their labels. However, manufacturers are responsible for making sure their products are safe.”
In addition to the fact that the FDA considers the expiry date to be the responsibility of the supplier, OTC drugs must be stability tested and labelled with an expiry date.
Author
Biorius
Related Posts
Get in Touch
Need a piece of advice, a quotation or answers to your questions?
Fill in this form or contact us directly: info@biorius.com – We will answer as soon as possible!
As specialists in cosmetic Regulations for more than 15 years, Biorius offers a reliable turnkey solution for placing cosmeticproducts in various markets:
-
First Class specialists in cosmetic regulations in Europe, the United Kingdom, the United States, and in more than 60 countries -
50 regulatory experts, toxicologists, pharmacists, and chemists to serve you. -
A unique model that guarantees you both the fastest turnaround possible and high-quality services. Curious? Ask us to know more! -
A best-in-class IT tool, free of charge, and saving a lot of your time. -
More than 1,500 international clients have already chosen Biorius! -
We evaluated more than 100,000 products and have always successfully managed compliance over 15 years of existence
Get in Touch
"*" indicates required fields
